Turn Stress into Simplicity
Everyone wants to reduce site burden, but conducting a study can be stressful. When studies are complex, both sites and patients struggle with adherence and lose interest, leading to data quality issues, poor enrollment, and low retention. Our platform centralizes tasks, technologies, and instructional content, providing clear guidance to sites and patients—simplifying even the most complex studies.
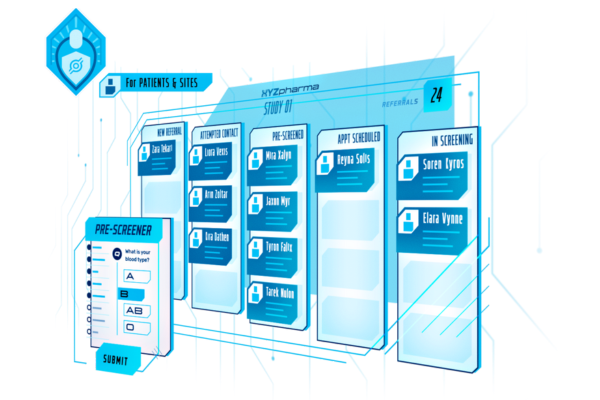
One Platform, Equal Support for Sites and Patients
We help sponsors deliver a smoother trial experience for both sites and patients. By simplifying complex visits through visit specific overviews, instructional content, and seamless tech integration, we reduce site burden and improve patient experiences. The result? A 40% increase in site compliance and better outcomes for everyone.
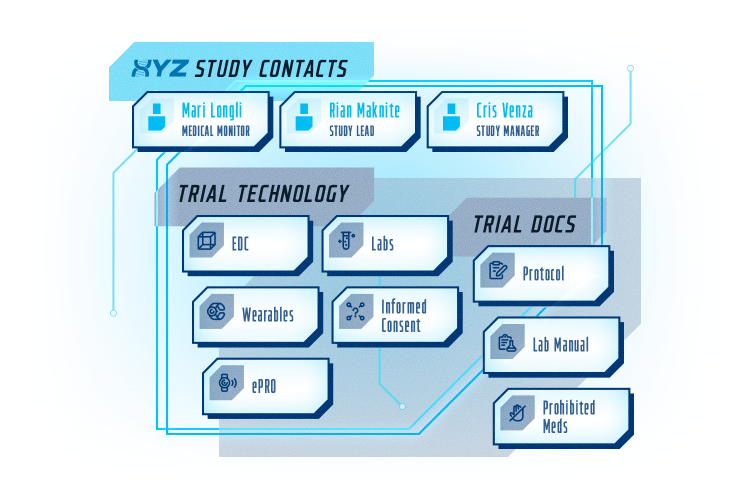
SITE HUB
CENTRALIZED ON DEMAND ACCESS TO EVERYTHING
Automate sharing of study information via our CTGOV integration. Provide key study team contacts and all study documents with version control. Organize all study technology, educational content, and important updates all in one place.
BOOSTING SITE SUCCESS WITH KNOWLEDGE SHARING
Gather and deliver important insights with study newsletters, enrollment updates, best practices, and site surveys - optimize not just site performance throughout the trial, but yours as well.
.png?width=750&height=500&name=patient%20guidance%20(1).png)
PATIENT HUB
FULLY INTEGRATED PATIENT SUPPORT
A personalized, comprehensive digital concierge to keep participants engaged and adherent with all tasks and tech throughout their clinical trial journey. By providing DEI-appropriate information tailored to each patient, this tool ensures successful participation and can even deliver trial summaries and individual results.
STEP-BY-STEP GUIDANCE FOR BETTER PATIENT COMPLIANCE
Patients often need reminders or clear instructions to stay on track during trials. Our tool provides step-by-step support throughout each stage, reducing confusion and ensuring adherence to study protocols, ultimately improving patient engagement and compliance.
BECAUSE SOMETIMES YOU JUST NEED A HUMAN TOUCH
Patients often face challenges like transportation and financial concerns that hinder their participation in clinical trials. Our patient support provider solution offers assistance with these issues, helping patients stay engaged and focused on the study, improving retention and overall success.
Say goodbye to site burden and hello to a smoother trial experience!
The Clinical Trial Experience Platform
Our platform centralizes and simplifies recruitment, conduct, and communication for sponsors, patients, and sites across the clinical trial experience.
ADMIN PORTAL
- Centralized platform for managing site operations and monitoring patient activities
- Analytics dashboard with insights into recruitment, retention, and compliance
- Tracks patient behavior, survey responses, and engagement metrics for informed decisions
ETMF INTEGRATION
- Seamlessly integrates with eTMF for secure storage and management of essential study documents
- Ensures compliance with regulatory requirements for document handling and storage
STUDY DOC ASSISTANT
- An AI-powered assistant streamlines document organization, version control, and retrieval
- Reduces administrative burden by automating document management
VISIT OVERVIEW TEMPLATES
- Develop detailed, compliant visit plans to ensure all study protocols are followed accurately
- Centralize instructions to streamline workflows, improving clarity and efficiency for study teams
- Enhance coordination and reduce errors by providing a unified platform for managing visit schedules and requirements
VENDOR TECH BOOKMARKS
- Provide on demand access to 3rd party technology links for essential systems and vendors
- No development required for 3rd parties. Just type in the study specific URLs and go
WHAT OUR CLIENTS SAY
ProofPilot has taken a more holistic approach without being a heavy weight “platform” and frankly it’s refreshing!
I really appreciate this portal. This scheduling method works best for me because I see patients during the day and sometimes the schedule varies.
I would love to use ProofPilot in other studies. It is much more efficient for contacting referrals, especially when you have multiple studies going on
This would be so helpful if patients from every study was using it!
Through integrated workflows to ensure timely training, logistics and patient payments, we saw nearly 90% compliance from our participants
We used PROOFPILOT to enroll 300 patients for DCT digital biomarker study – it delivered enormous cost savings, quality data, and great compliance.



